Miquel Vila-Perelló: "A few years ago, when you spoke to international investors and told them you were from Barcelona, they gave you the cold shoulder."
Co-founder and CEO of SpliceBio


BarcelonaSpliceBio has become the biotechnology company that has raised the most money in the history of the sector in Spain. From its laboratories in the Barcelona Science Park (PCB), the Catalan company has raised €119 million. in a financing round which will be used to validate the gene therapy he has developed for a rare disease that causes blindness. More than ten years ago, the company was born from the research conducted by Miquel Vila-Perelló and Silvia Frutos in the United States, under the leadership of Professor Tom Muir, a pioneer in protein modification that allows them to attack Stargardt disease. With a workforce of more than 40 employees, the company grew alongside the local biotechnology sector, which set another investment record this first half of the year with €227 million, according to data from Biocat.
With a degree and PhD in organic chemistry from the University of Barcelona (UB), Vila-Perelló (Barcelona, 1977) arrived in the United States in 2005. First in New York, at Rockefeller University, where he joined Muir's research group. Then it was time for Princeton. He ended up spending ten years of his life on the other side of the ocean, where there are many more resources for companies like his, but in 2014 he wanted to return home to contribute to a biotechnology sector that was just starting to take off by launching SpliceBio, initially named ProteoDesign.
How do you handle the responsibility of having closed the largest round in the history of the sector in Spain?
— We hope there will be more. Rounds like this show international investors that doing this in Barcelona is possible. It's not just about the money you raise, but the group of investors you attract. In our case, we have venture capital funds from pharmaceutical companies like Roche, Sanofi, Novartis, and UCB, but also EQT, one of the largest funds in Europe, and New Enterprise Associates (NEA), one of the largest in the United States. The fact that firms like these decide to invest in a Barcelona company built with local talent should make us feel inferior, because it shows us that this can be done from here. A few years ago, when you went to talk to international investors and told them you were from Barcelona, they would give you dirty looks.
And now?
— A little less. The city is starting to position itself on the map, but there's still a long way to go. Many of these international investors are unfamiliar with the Spanish legal system. It's somewhat different from how it works in Anglo-Saxon countries, and there are some things that shock them a bit. Investors are afraid of anything they don't know, and the best thing to do when faced with fear is to say no. For all these investors who have now invested in SpliceBio, the next time they need to invest in another Catalan or Spanish company, it will be much easier, because there are some problems they've already resolved with us. As also happened when they invested in other biotech companies like Minoryx or Sanifito. The more often it happens, the less that barrier will be. In this sense, Ysios Capital has been doing a great job participating in international rounds, and Asabys Partners has also continued to do so. This has allowed them to connect with these foreign investors and has made it easier for them to come here later.
What is the city missing?
— The circle needs to be completed. Now we have the idea, the investment, and the company's growth. The biotechnology sector has been able to develop here thanks to the quality science emerging from research centers, the growth of venture capital in Barcelona, and the entrepreneurial spirit. But, in the case of companies funded by venture capital, investors need to recoup their money. Developing drugs costs a lot of money and carries a very high risk. Acquisitions are necessary. We have examples like the acquisition of the Barcelona-based company. Stat-Dx, but more examples like this are needed. We also need drugs developed here to reach the market. Avizorex Pharma has achieved this. But one flower doesn't make a summer; more are needed. We need to incentivize investors to see that investing in local companies pays off, but also so that young people see that there are careers here with a future. It's hard to attract talent now because if you go to work in a start-up from London or Boston and it closes, you have fifty more there to find a new job.
And how do you solve this?
— With a little patience. Developing a drug takes a long time. On average, about ten years. And the chances of success are 10%. Here, the sector began to take off in 2014, and we're reaching the point where these things should happen. Therefore, there's a lot of criticism needed. More biotech companies must be created so that one will eventually reach the market. Investors who have the patience to wait and see the fruits of these investments are also needed. The healthcare sector has a very laudable goal: developing drugs to improve people's lives, but investors are investing money at risk, and it's reasonable for them to expect a financial return.
The city is now attracting large companies like AstraZeneca.
— Investments such as the new pharmaceutical center help position Barcelona on the map. Sanofi It also plans to increase its presence in the city. All of this contributes to attracting more talent. Politicians often talk about how important this sector is, but perhaps not much is done, and I think this type of commitment is what makes the sector valued. It helps people see that here there is the possibility of earning a good living in sectors that contribute to society, and that you can earn a good living not only by being one. influencerIt would have been fantastic if the European Medicines Agency (EMA) had been based in Barcelona. Failing that, let AstraZeneca and everyone else come.
What has SpliceBio achieved?
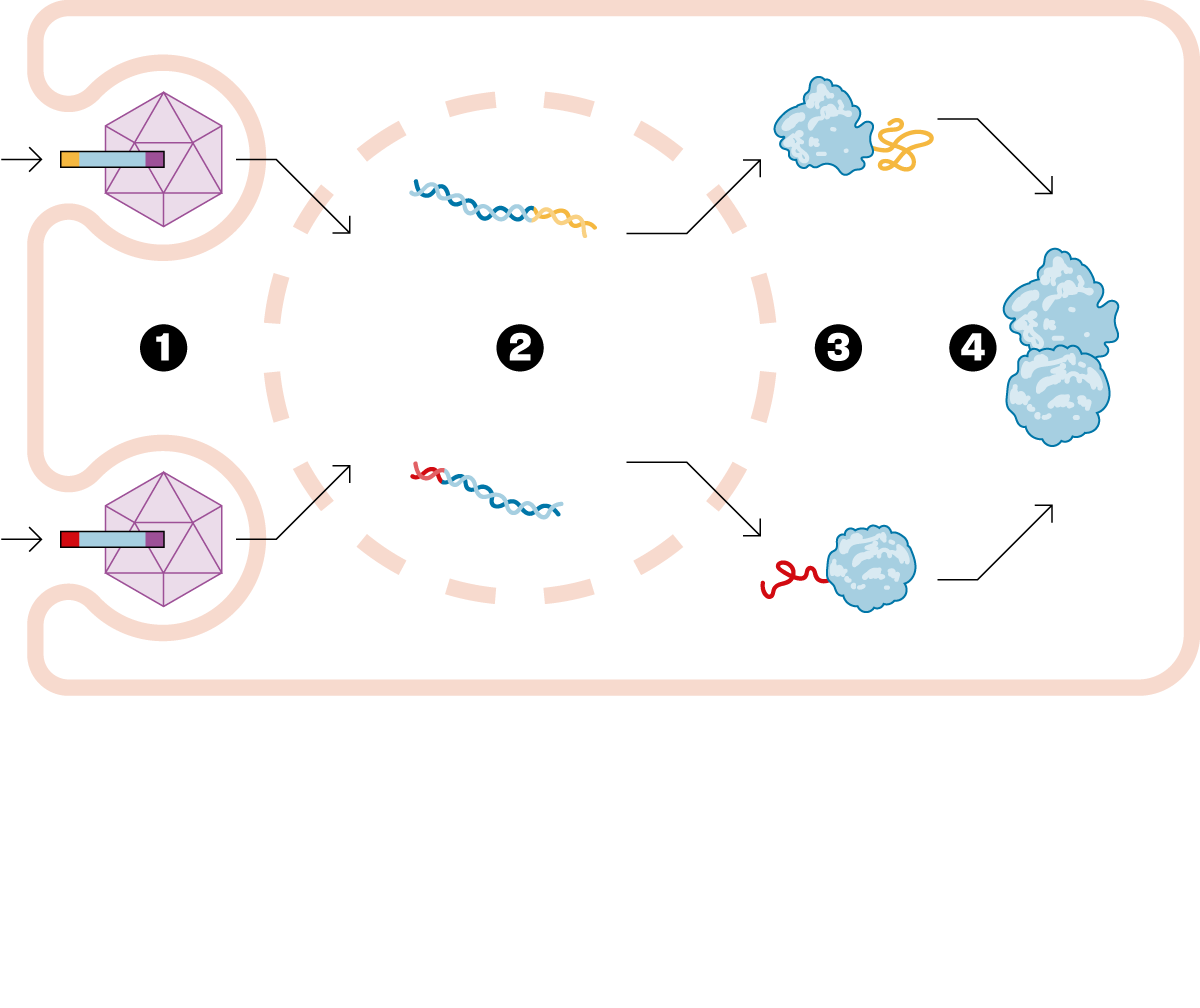
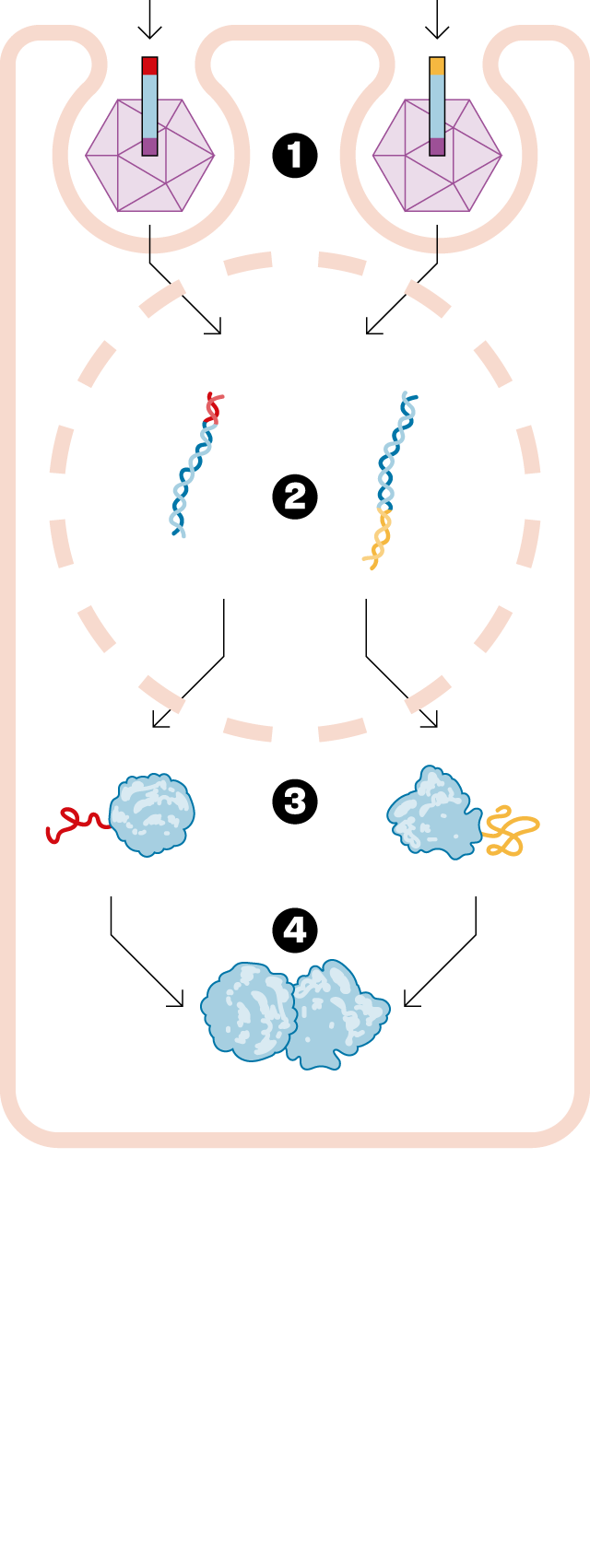
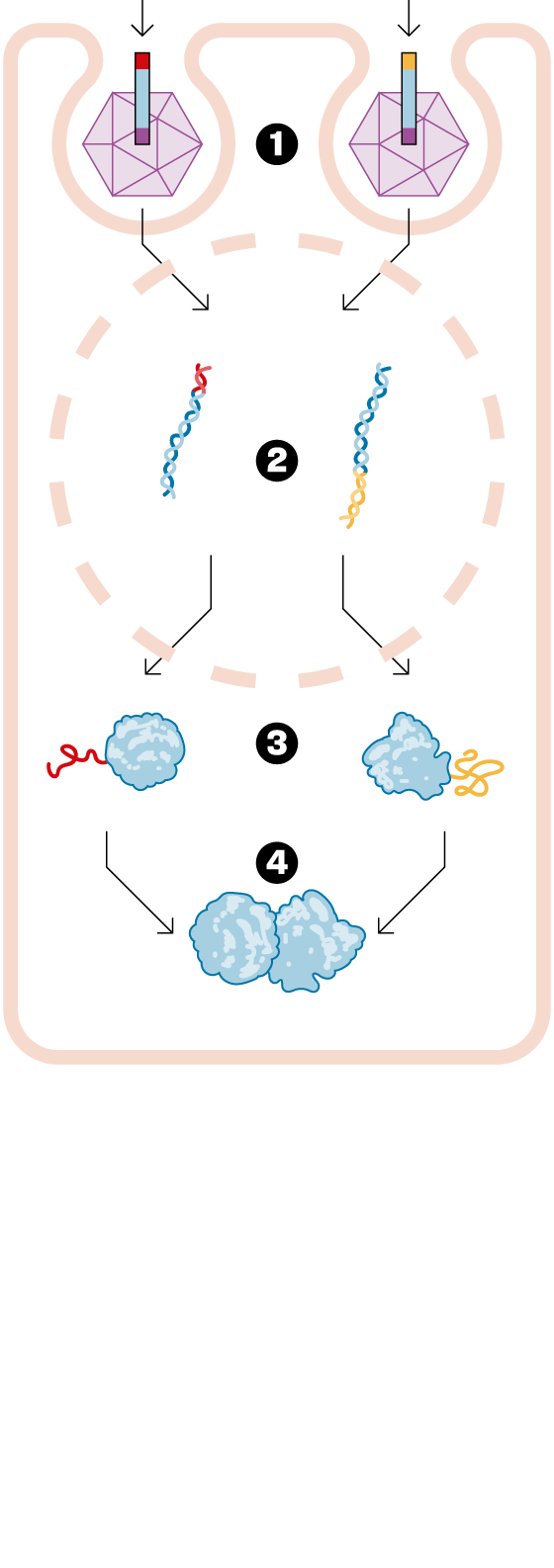
— There are diseases caused by a gene with a mutation that causes it to malfunction. Specifically, it doesn't make the protein it's supposed to make, since genes are dedicated to making proteins. Gene therapy involves designing a drug that delivers a good version of this diseased gene, thus curing the disease. Of the thousands of approved drugs, only six are gene therapies. It's a very novel field. Until now, traditional gene therapy has been able to inject a small gene. The problem is that many of these genes to be replaced are too large. The technology we developed at Princeton allows this gene to be delivered in two fragments. Once delivered to the patient, the entire protein needed to cure them is formed. We've opened up a new world within gene therapy, and we can now consider treating a whole range of diseases that were previously incurable.
How did he do it?
— To add the good gene to the body, a virus that has been emptied of its bad part is used. But only up to a certain size can fit inside this virus. Our solution is to split the gene into two parts and insert each piece into a different virus. This is our drug, and when we inject it, each piece of the gene is released and joins together to form the entire protein needed. This technology is called, in English, protein splicingHence the name SpliceBio. Splicing can be translated as splice either paste. It's in the field of gene therapy that this technology can truly be transformative, allowing us to do things that weren't possible otherwise.
How can a virus be used to cure a disease?
— The viruses we use are a very specific type, called adeno-associated viruses. First of all, they cannot reproduce, and therefore, they are not dangerous to human health. They simply infect the cell into which they are injected, deliver the good gene, and then disintegrate. In fact, they function like a box containing only the genetic material.
He has decided to focus on genetic diseases that affect the eyes.
— Ophthalmology allows for localized injection of the treatment, meaning you don't have to expose the rest of the body to the therapy, which reduces the drug's risks. The eye is considered an immunoprivileged organ because it is highly isolated from the rest of the body. We are focusing on Stargardt disease, which affects the retina. It manifests between the first and second decades of life, and patients become progressively blind. It is hereditary and has no treatment. It is a rare disease, but within that, it affects a large number of patients—about 100,000 voters between Europe and the United States—and currently there is no treatment for it.
What phase is the drug in?
— The objective of the round we just closed is to fund the clinical trial for Stargardt disease. We announced the treatment of the first patient earlier this year. We've started in the United States and the United Kingdom, and we're working to bring it to a European country. We decided to do so due to regulatory difficulties in Europe. In the United States, when you apply for authorization from the FDA, you suddenly have access to 350 million patients. In Europe, on the other hand, you have to request authorization from each country. In our case, with a rare disease, one country isn't enough, and that forces you to go to multiple countries, which greatly complicates the process.